Document Management System In Pharma
Document management system in pharma. Cloud-based tools include document management training CAPA validation and more. This document management software is for Startups SMEs Agencies Enterprises and can be deployed on Any. Head- QA shall be accountable for compliance of this SOP.
Ad Search for Jobs with High Incentives for various industries across desired Location. AmpleLogic EDMS Software is a power-packed electronic document management system exclusively designed for Pharmaceutical and Biotech companies developed and implemented exclusively to meet the cGMP needs of Pharma domain with 21CFR Part 11 compliance EU Annex 11 compliance can easily eliminate all the challenges faced by different companies. Applicable for Document Management system in Quality Assurance department Responsibility.
To lay down a procedure for Management of Documents in Quality Assurance Department in Pharma Industry. Prepare for risk management through essential activities critical to design and implementation of a risk management program. Document Management System in Quality Assurance Department Objective.
To lay down a procedure for Management of Documents in Quality Assurance Department. Quality Assurance Department Accountability. 5 reviews Visit Website.
Delivered in the cloud Vault enables you to manage content needs across your entire organization-and around the globe. It is a configurable and easy-to-use software solution that helps pharmaceutical companies attain and sustain cGMP compliance. The SOP is applicable for policy documents related to quality Management systems and also provides guideline for numbering of validation protocols and reports.
The functionality built into the pharma READY Document Management system stems from a deep understanding of the requirements for meeting and maintaining compliance with FDA 21 CFR Part 11 and other international standards. The Pharma IT Cloud EDMSQMS is a complete GxP-validated Document Quality and Training Management system based on Ennov software and pre-configured according to industry best practices. Crown Document Management is a document management system for Pharmaceuticals industry.
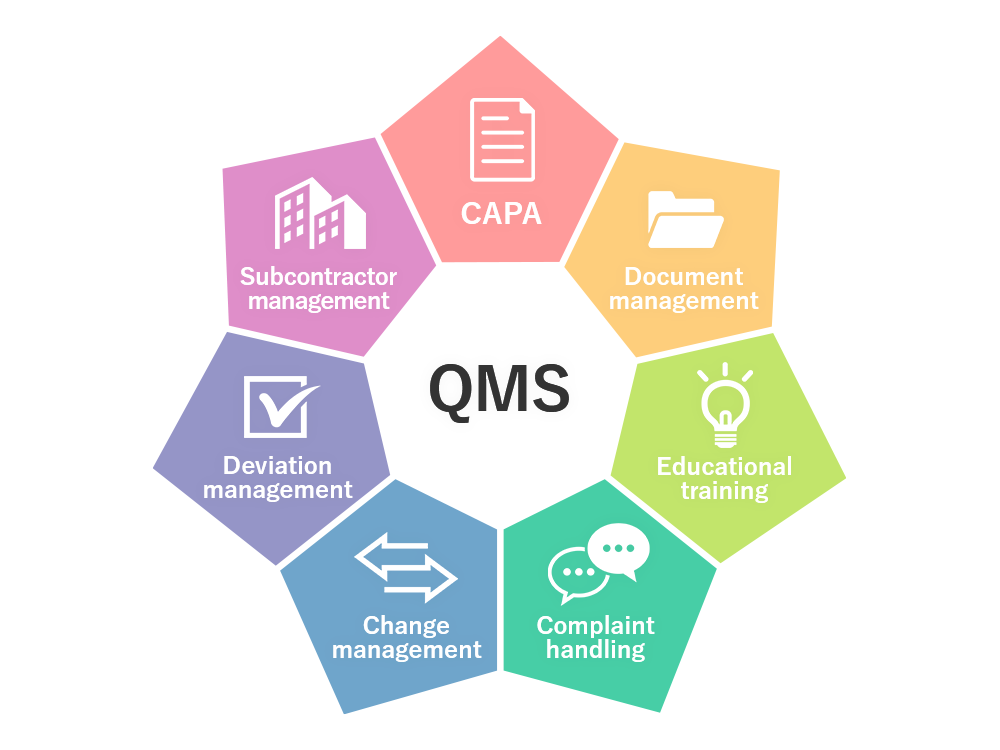
Below the Standard Operating System SOP for Document Management System. Applicable for Document Management system in Quality Assurance department.
Applicable for Document Management system in Quality Assurance department.
Todays top 10 high paying Job roles. Ad Get the Management Systems your competitors are already using - Start Now. Head- QA shall be accountable for compliance of this SOP. Documentation is the key to GMP compliance and. Document Management System Law Firm. Cloud-based tools include document management training CAPA validation and more. With industry-specific applications for regulatory clinical quality medical and commercial Veeva Vault is a complete content management system. It is a configurable and easy-to-use software solution that helps pharmaceutical companies attain and sustain cGMP compliance. A document is a physical or digital representation of a body of information designed with the capacity and usually intent to communicate.
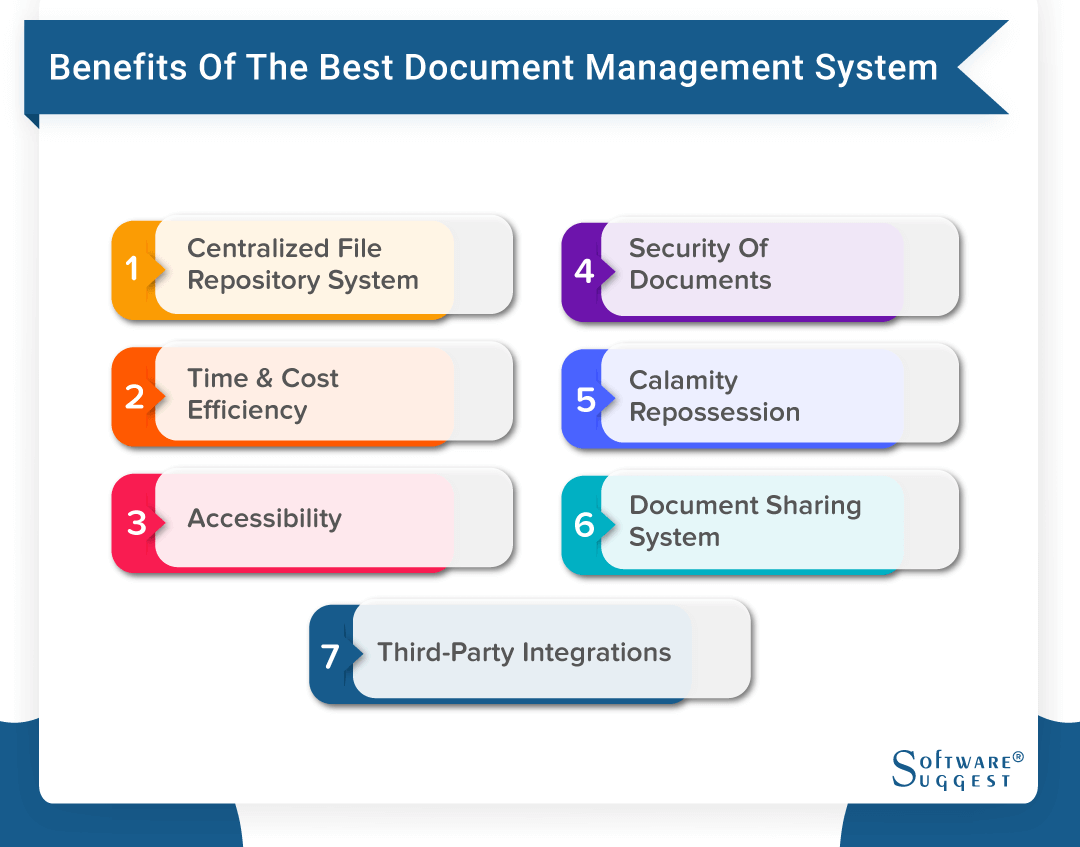
Head- QA shall be accountable for compliance of this SOP. Choose Your Management Systems from the Premier Resource for Businesses. It is popularly said that Anything which is not documented is not done. AmpleLogic EDMS Software is a power-packed electronic document management system exclusively designed for Pharmaceutical and Biotech companies developed and implemented exclusively to meet the cGMP needs of Pharma domain with 21CFR Part 11 compliance EU Annex 11 compliance can easily eliminate all the challenges faced by different companies. Applicable for Document Management system in Quality Assurance department. Documentation is the key to GMP compliance and. Pharmaceutical and Biotech companies are challenged to meet control documentation requirements in day to day operationsEffective document management and document control are critical parts to any quality and compliance solution.





























Post a Comment for "Document Management System In Pharma"